Structure and bonding in Au(i) chloride species: a critical examination of X-ray absorption spectroscopy (XAS) data†
Abstract
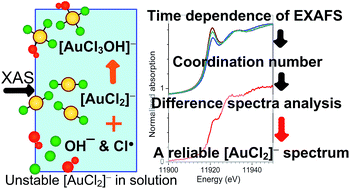
Au(I) chloride species are important reactants and intermediates in various processes across the chemical sciences and engineering. Structure and bonding in Au(I) species are often characterized by X-ray absorption spectroscopy (XAS), including measurements under reaction conditions. Previously reported XA spectra for Au(I) chloride species have varied significantly, likely as a result of radiation damage and/or partial disproportionation of [AuCl2]− ions, which are metastable under ambient conditions. By monitoring the decomposition of tetrabutylammonium dichloroaurate(I), TBA[AuCl2], in 1,2-dichlorobenzene we have obtained a reliable X-ray absorption spectrum of [AuCl2]− ions by combining the calculation of difference spectra with an extended X-ray absorption fine-structure (EXAFS) determination of the solution composition. The results show that the X-ray absorption near-edge structure (XANES) of [AuCl2]− is characterized by a weak Au 2p3/2 → 5d (‘white line’) transition, which agrees well with the spectrum predicted by electronic structure calculations using the FEFF8 code. Compared to [AuCl4]−, the determined [AuCl2]− spectrum has several distinctive features of diagnostic analytical value. A more detailed densities of states (DOS) analysis of the electronic structure suggests that the weak white line arises from a hybrid Au 6s/5d DOS band that is partially occupied, up to the level of the highest occupied molecular orbital (HOMO). Correlation of Cl coordination numbers determined from the EXAFS with the intensity of the white line in the XANES indicates that the decomposition is a primarily radiation-induced oxidation to Au(III) species with an average formula of [AuCl3OH]−.

 Please wait while we load your content...
Please wait while we load your content...