Preparation, characterization, and catalytic application of nano Ag/ZnO in the oxidation of benzylic C–H bonds in sustainable media†
Abstract
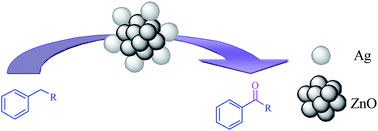
Nano Ag/ZnO is successfully synthesized by a new simple and low cost method employing Zn(NO3)2·6H2O, AgNO3, and urea. X-ray diffraction (XRD), transmission electron microscopy (TEM), adsorption/desorption porosimetry (BET) and X-ray photoelectron spectroscopy (XPS) were used to characterize the structure and morphology of the catalyst. The amount of Ag loading on ZnO (9.276 × 10−5, 7.391 × 10−5, 3.632 × 10−5, and 3.286 × 10−5 mol%) was determined by induced coupled plasma (ICP). The resulting ZnO-supported Ag nanoparticles efficiently catalyzed the oxidation reactions of alkyl-substituted benzenes with hydrogen peroxide (H2O2) as a cheap and environmentally friendly oxidant under solvent-free conditions. This catalytic reaction gave good to excellent yield of products by using mild reaction conditions and no additives were employed during the reaction.

 Please wait while we load your content...
Please wait while we load your content...