Unraveling the binding interaction of Toluidine blue O with bovine hemoglobin – a multi spectroscopic and molecular modeling approach†‡
Abstract
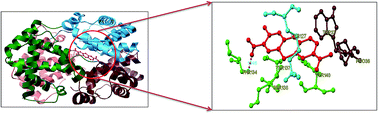
Toluidine blue O (TBO) is a cationic photosensitizer that belongs to the class of phenothiazinium dyes. It has been widely used in photodynamic therapy due to its preferential affinity towards cancer cells in vivo. The present study reports a detailed binding mechanism of photodynamic therapeutic agent TBO with bovine hemoglobin (BHb) using steady state emission, time resolved fluorescence, UV-Vis absorption, circular dichroism (CD) and three dimensional emission (3D) spectral studies. The results from the steady state emission and time resolved fluorescence studies revealed that the quenching of BHb emission by TBO is due to the formation of a ground state (BHb–TBO) complex with a substantial binding affinity value of (1.19 ± 0.30) × 105 dm3 mol−1. Moreover, TBO induced tertiary and secondary conformational changes of BHb were monitored by UV-Vis absorption, CD and 3D emission spectral studies. The most probable binding location of TBO within the cavity of BHb has been investigated by AutoDock-based blind docking simulation. The negative Gibbs energy change and docking results revealed that hydrogen bonding and hydrophobic interactions played a crucial role in the complexation of TBO with BHb.

 Please wait while we load your content...
Please wait while we load your content...