EGFR/HER-2 inhibitors: synthesis, biological evaluation and 3D-QSAR analysis of dihydropyridine-containing thiazolinone derivatives†
Abstract
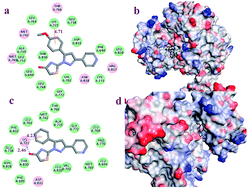
A series of dihydropyridine containing thiazolinone derivatives (4a–4r) have been designed, synthesized and their biological activities evaluated as potential EGFR and HER-2 kinase inhibitors and in tumor cell antiproliferation. The synthesized compounds were analyzed by 1H-NMR and MS. In addition, compound 4m was scrutinized by X-ray structure analysis. Among them, compound 4r displayed the most potent inhibitory activity (IC50 = 0.099 μM for EGFR and IC50 = 3.26 μM for HER-2). Antiproliferative assay results indicated that compound 4r owned high antiproliferative activity against B16–F10, HeLa and MCF-7 in vitro, with IC50 values of 0.09 μM, 0.29 μM, and 0.56 μM, respectively. Docking simulations were further performed to position compound 4r into the EGFR active site to determine the probable binding mode. 3D-QSAR models were built for reasonable design of EGFR/HER-2 inhibitors in the present and the future.

 Please wait while we load your content...
Please wait while we load your content...