Cation distribution, structural, morphological and magnetic properties of Co1−xZnxFe2O4 (x = 0–1) nanoparticles
Abstract
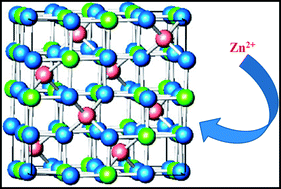
Co1−xZnxFe2O4 (x = 0.0, 0.2, 0.5, 0.6, 0.8, 1.0) nanoparticles (NPs) are prepared by a wet chemical co-precipitation method. The presence of zinc ions causes appreciable changes in the structural and magnetic properties of the Zn-substituted CoFe2O4. The structural, morphological and magnetic properties of the samples are determined and characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), and vibrating sample magnetometry (VSM). The particle size measured from TEM and XRD patterns confirms the nanosized dimension of the NPs in the size range of 9.0–15 nm. The saturation magnetization and the experimental magnetic moment are observed to initially increase (up to x = 0.2), which is explained by Néel's collinear two-sublattice model, and then continuously decrease with further increase in Zn content x. This decrease obeys the three-sublattice model suggested by Yafet–Kittel (Y–K). The Y–K angle is zero for the CoFe2O4 NPs, it increases gradually with increasing Zn concentrations and can be extrapolated to 79.71 for ZnFe2O4 NPs.

 Please wait while we load your content...
Please wait while we load your content...