Rhodium catalysed conversion of carbenes into ketenes and ketene imines using PNN pincer complexes†
Abstract
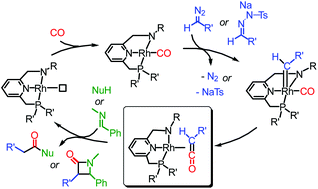
Ketene synthesis involving catalytic carbonylation of carbenes is an interesting alternative to traditional synthetic protocols, offering milder conditions to diversified products. Analogous catalytic ketene imine production from carbenes and isocyanides is also a promising reaction. However, both methods are underdeveloped. Rhodium carbonyl complexes B and E, based on (6-(phosphinomethyl)pyridin-2-yl)methan-sec-amine type PNN ligand scaffolds, reveal good catalytic activities in ketene and ketene imine production using ethyl diazoacetate (EDA, 1) or sodium 2-benzylidene-1-tosylhydrazin-1-ide (5) as the carbene precursors, as demonstrated by in situ amide/imidamide and β-lactam synthesis. DFT calculations suggest that diazo activation is the rate-determining step and that NH-deprotonation of the ligand produces a more active rhodium complex. The ketene formation step likely proceeds via an outer-sphere CO insertion mechanism. Subsequent stepwise and concerted [2 + 2] cyclization mechanisms have comparable barriers. The complexes are the first rhodium catalysts reported for catalytic ketene/ketene imine production from carbenoids. The higher affinity of rhodium for binding ketene or ketene imine intermediates as compared to other reported metal catalysts (i.e. Pd, Co) may provide opportunities for future enantioselective reactions when using chiral ligands.
- This article is part of the themed collections: Carbenes for Today and HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...