Pincer CNC bis-N-heterocyclic carbenes: robust ligands for palladium-catalysed Suzuki–Miyaura arylation of bromoanthracene and related substrates†
Abstract
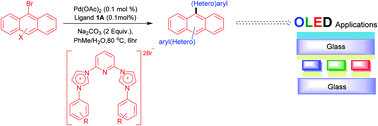
Novel pincer-type N-heterocyclic carbene ligands have been synthesised and characterised by FAB-MS and single crystal X-ray analysis. The ligands have proved to be highly active towards palladium-catalysed Suzuki–Miyaura cross-coupling of bromoanthracene and related substrates allowing the efficient and easy access of molecules for possible OLED applications.

 Please wait while we load your content...
Please wait while we load your content...