Palladium-catalyzed alkylation of unactivated C(sp3)–H bonds with primary alkyl iodides at room temperature: facile synthesis of β-alkyl α-amino acids†
Abstract
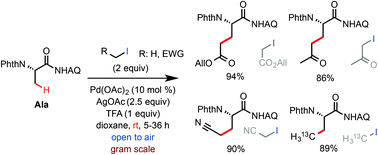
An efficient protocol for the synthesis of β-alkyl α-amino acids via palladium-catalyzed β-C(sp3)–H alkylation of aminoquinoline-coupled phthaloyl alanine is developed. The new TFA-promoted reaction conditions provide excellent C–H alkylation reactivity with alkyl iodides bearing moderately electron-withdrawing groups at room temperature. A range of β-alkyl α-amino acid products, including some difficult to access by other means, can be quickly prepared from readily available primary alkyl iodides and alanine precursors.

 Please wait while we load your content...
Please wait while we load your content...