Abstract
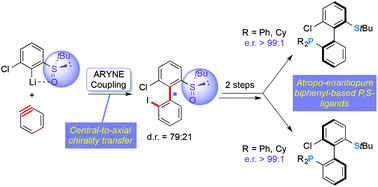
The first atropo-selective aryl–aryl coupling based on arynes in the presence of a tert-butylsulfinyl group as the chiral auxiliary on the aryllithium nucleophile is described. The approach allows for the efficient access to a novel family of atropo-enantiopure biphenyl-based phosphine–thioether ligands. The new P,S heterodonor ligands were assessed in model palladium-catalyzed allylic substitution reactions.
- This article is part of the themed collection: Celebrating the 80th Birthday of Professor Ei-ichi Negishi


 Please wait while we load your content...
Please wait while we load your content...