N-Alkyl ammonium resorcinarene salts: multivalent halogen-bonded deep-cavity cavitands†
Abstract
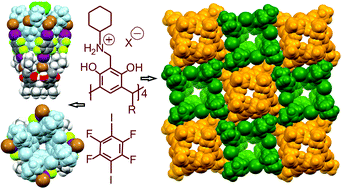
N-Cyclohexyl ammonium resorcinarene halides, stabilized by an intricate array of hydrogen bonds in a cavitand-like assembly, form multivalent halogen-bonded deep-cavity cavitands with perfluoroiodobenzenes. As observed from the macromolar to infinite concentration range through crystal growth and single crystal X-ray analyses, four 1,4-diiodotetrafluorobenzenes form moderate halogen bonds with the bromides of the N-cyclohexyl ammonium resorcinarene bromides leading to a deep-cavity cavitand-like structure. In this assembly, the N-cyclohexyl ammonium resorcinarene bromide also acts as a guest and sits in the upper cavity of the assembly interacting with the 1,4-diiodotetrafluorobenzene through strong π⋯π interactions. Solvent molecules act as guests and are located deep in the cavity of the resorcinarene skeleton. In the millimolar range, 1H and 19F NMR spectroscopic analyses confirm halogen bonding in solution. Fast exchange binding of electron rich fluorophores (naphthalene, anthracene and pyrene) in the upper layer of these assemblies was also observed in the millimolar range while in the micromolar range, using fluorescence analysis, no binding of the fluorophores was observed.

 Please wait while we load your content...
Please wait while we load your content...