Asymmetric boron conjugate addition to α,β-unsaturated carbonyl compounds catalyzed by CuOTf/Josiphos under non-alkaline conditions†
Abstract
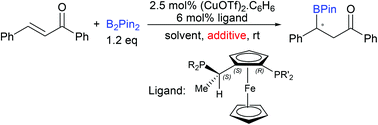
The asymmetric boron conjugate addition onto α,β-unsaturated ketones and esters has been developed by using the CuOTf/Josiphos complex as the catalyst under non-alkaline conditions. It was found that the addition of MeOH into the reaction system is crucial to the catalytic reactivity. Good to excellent enantioselectivity (up to 96% ee) and yields (up to 98%) have been achieved for 15 examples.

 Please wait while we load your content...
Please wait while we load your content...