Combining coordination and supramolecular chemistry to explore uranyl assembly in the solid state†
Abstract
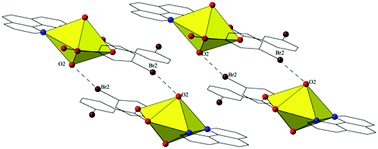
The syntheses and crystal structures of twelve new compounds containing the UO22+ cation, a bromo-substituted benzoic acid linker (m-bromo-, p-bromo, or 3,5-dibromobenzoic acid) and a chelating N-donor (1,10-phenanthroline, 2,2′:6′,2′′-terpyridine, or 4′-chloro-2,2′:6′,2′′-terpyridine) are reported. Single crystal X-ray diffraction analyses of these materials allowed for the exploration of the structural relationship between the benzoic acids and the chelating N-donor, as well as the influence of pH on uranyl speciation. At an unadjusted pH (∼3) a mix of uranyl monomers and dimers are observed whereas at higher pH (5–6) uranyl dimers are usually produced with monomers and tetramers also observed. A systematic study of the supramolecular interactions present in these materials was executed by varying the bromine position on the benzoic acid groups along with substituents on the chelating N-donor. Assembly via halogen and hydrogen bonding interactions as well as π–π interactions, including four instances of uranyl oxo-functionalization via halogen bonding, was observed depending on the experimental conditions utilized.
- This article is part of the themed collection: Crystal engineering for molecular materials

 Please wait while we load your content...
Please wait while we load your content...