Effect of electrolyte concentration on uranium species adsorption: a molecular dynamics study
Abstract
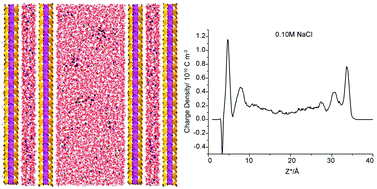
Molecular dynamics (MD) simulations of uranyl species adsorption in montmorillonite pores in 0, 0.05 and 0.10 M NaCl were carried out to investigate the influence of internal electrolyte concentration on the uptake amount, species distribution and electrical double-layer (EDL) structures. Most cases revealed that the β- and d-planes of the adsorbates are located 4.3–4.6 Å and 5.5–5.8 Å from the clay planes, respectively. However, based on the split carbonate peaks, an exception, the left peaks near the clay surface for 0.10 M NaCl, formed more uranyl-(bi)carbonate complexes than the other peaks. Under this condition, each peak and minimum of cations shifted slightly farther away from the clay plane. For this outlying case, the charge density profile confirmed the charge inversion of carbonates near like-charged surfaces. Collectively, the simulations revealed the subtle influence of the internal NaCl concentration (0–0.05 M) on the EDL structure, uptake amount and species distribution. In particular, a threshold concentration (0.10 M NaCl in this study) for charge inversion within the β-plane may exist. Under this condition, a pronounced change in the EDL structure occurs, which in turn causes a dramatic alteration in the uranyl species adsorption relative to lower electrolyte concentrations.
- This article is part of the themed collection: Celebrating 110th Anniversary of Chemistry at Peking University


 Please wait while we load your content...
Please wait while we load your content...