Solvent-free mechanochemical synthesis of a bicyclononyne tosylate: a fast route towards bioorthogonal clickable poly(2-oxazoline)s†
Abstract
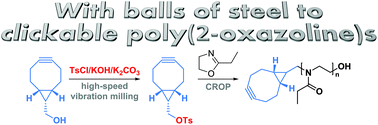
The mechanochemical synthesis of a bicyclononyne tosylate (BCN-OTs) is presented. BCN-OTs is demonstrated to be a good initiator for the cationic ring-opening polymerization of 2-ethyl-2-oxazoline directly yielding BCN functional poly(2-ethyl-2-oxazoline) (PEtOx-BCN) with high chain end fidelity. Subsequent strain-promoted cycloadditions of the resulting PEtOx-BCN enable efficient additive-free conjugation reactions as demonstrated for the formation of a block copolymer and a PEtOx–protein conjugate.

 Please wait while we load your content...
Please wait while we load your content...