Programmable self-assembly of a cystamine-block copolymer in response to pH and progressive reduction–ionization–oxidation†
Abstract
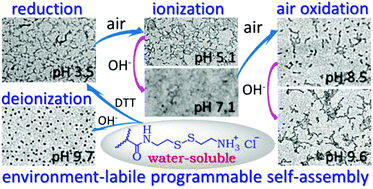
Direct aqueous synthesis and programmable self-assembly and reconstructions of a well-defined reactive cystamine-block copolymer in response to conditions of the aqueous environment, such as air and solution pH, are presented in this article. A variety of well-defined poly(cystamine methacrylamide hydrochloride) (PCysMA) polymers and copolymers with 2-hydroxypropylmethacrylamide (HPMA) and 2-aminoethylmethacrylamide hydrochloride (AEMA) can be achieved via fast and well-controlled aqueous RAFT under visible light irradiation at 25 °C. PHPMA-b-PCysMA assembles into spherical PCysMA-core micelles upon NH3+-to-NH2 conversion. Moreover, progressive reactions in the PCysMA block, including reduction, ionization and oxidation, can be induced stepwise by reduction in argon gas saturated acidic water, exposure to air and stepwise alkalization. These reactions lead to the stepwise conversion of the intermolecular interactions from electrostatic repulsion (ionized PCysMA block) into hydrogen-bonding associations (reduction-generated thiol block), electrostatic repulsion (as-ionized thiolate block), and hydrophobic cross-linking (oxidation-generated disulfides), leading to the programmable self-assembly and reconstruction of a water-soluble block copolymer into compound-encapsulated nanowires, compound-released nanowires, shortened nanorods/spheres, swollen nanowires, nanorods, and branched nanowires/networks. All the phase transformations stem from the environment-mediated reaction complexity of the CysMA unit, and hold potential for biological and other emerging fields.

 Please wait while we load your content...
Please wait while we load your content...