Aqueous copper(0) mediated reversible deactivation radical polymerization of 2-hydroxyethyl acrylate†
Abstract
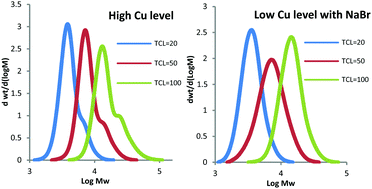
Reversible deactivation radical polymerization (RDRP) of 2-hydroxyethyl acrylate in D2O with Cu(0) wire mediation and with two-step Cu(0) in situ mediation was investigated. The concentration of active species on the Cu(0) surface, which was influenced by Cu(0) type (wire or particle), polymer chain length, and activator and deactivator concentration, was the key factor in understanding the formation of insoluble gel with Cu(0) wire and/or a high molecular weight (MW) shoulder observed in the polymer molar mass distributions (MMDs). The influences of temperature, residual oxygen (dependent on the transfer procedure used to add reagents), and NaBr addition were also studied. The insights gained were used to produce P(HEA) with high molar mass [target chain length (TCL) = 400, 87% conversion, Đ = 1.16] using only ca. 250 ppm copper at room temperature, the first reported preparation of high MW P(HEA) with a low Đ using such a low copper catalyst concentration in a purely aqueous environment.

 Please wait while we load your content...
Please wait while we load your content...