One-pot two polymers: ABB′ melt polycondensation for linear polyesters and hyperbranched poly(ester-urethane)s based on natural l-amino acids†
Abstract
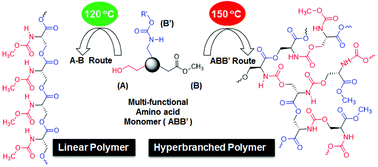
We report a novel one-pot ABB′ synthetic route for linear polyester and hyperbranched poly(ester-urethane)s based on multi-functional L-amino acid monomers via a temperature selective melt polycondensation approach. L-Serine, D-serine and L-threonine amino acids were converted into multi-functional ABB′ monomers (A = hydroxyl, B = carboxylic ester and B′ = urethane). At 120 °C, the ABB′ monomer underwent thermo-selective transesterification polycondensation (A reacted with B) to produce linear polyesters with B′ functionality as the pendent functionality in each repeating units. At 150 °C, the ABB′ monomer underwent dual ester-urethane self-polycondensation to produce new classes of hyperbranched poly(ester-urethane)s (A reacted with B and B′). Interestingly, the secondary hydroxyl group in the L-threonine monomer did not react at 120 °C; however, it became active at 150 °C to yield exclusively linear polyesters. The temperature selective polycondensation process was confirmed by appropriate model reactions and 1H and 13C NMR spectroscopic analysis. The role of the macrocyclic formation in the polycondensation process was also investigated by MALDI-TOF MS. The amino acid based new polymers were found to exhibit diverse molecular self-assembly. The linear polyesters adopted a β-sheet conformation which produced a helical nano-fibrous morphology. The hyperbranched polymers underwent a globular coil-like conformation for spherical nano-particular assemblies. Both the secondary structure formation as well as their morphological features were confirmed by circular dichroism spectroscopy and electron and atomic microscopy analyses. The new one-pot synthetic pathway is versatile in making diverse linear and branched polymers based on natural L-amino acids with a nano-fibrous or a spherical morphology for future applications in biomedical and thermoplastic industries.

 Please wait while we load your content...
Please wait while we load your content...