Synthesis and properties of low band gap polymers based on thienyl thienoindole as a new electron-rich unit for organic photovoltaics†
Abstract
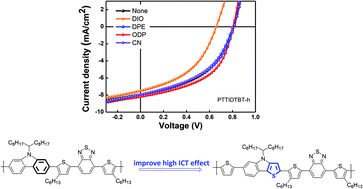
A series of polymers based on 6-(2-thienyl)-4H-thieno[3,2-b]indole (TTI), a new electron-rich unit for organic photovoltaics, was synthesized. By replacing the six-membered benzene ring of the carbazole with an electron-rich five-membered thiophene ring, an enhanced ICT effect between the electron-rich group and the electron-deficient group is expected to result in improved π-electron delocalization, low band gap and increased light-harvesting ability of the OPVs. The TTI unit, with a fused rigid backbone, has an efficient structure for the ICT effect and for tuning of the HOMO and LUMO energy levels to generate a low band gap. As electron-deficient units, 4,7-bis(4-hexylthiophen-2-yl)-2,1,3-benzothiadiazole (DTBT-h), 2-dimethyl-4,7-di(2-thienyl)-2H-benzimidazole (DTMBI) and 3,6-di(2-thienyl)-2,5 dihydropyrrolo[3,4-c]pyrrole-1,4-dione (DPP) were introduced by using Stille polymerization. PTTIDTMBI and PTTIDPP have low band gaps and show absorption up to 849 and 948 nm, respectively. The highest PCE was achieved with the device fabricated from a PTTIDTBT-h : PC71BM (1 : 3 w/w) blend with 1,8-octanedithiol (ODT) as an additive. The device demonstrated a Voc value of 0.81 V, a Jsc value of 8.19 mA cm−2, and an FF of 0.51, giving a highest power conversion efficiency of 3.35%.

 Please wait while we load your content...
Please wait while we load your content...