Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups†
Abstract
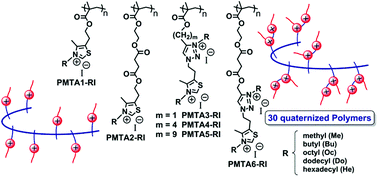
Polymers containing quaternary ammonium cations (QUATS) are well-known antimicrobial and disinfectant agents. Mono- and bis-heterocyclic methacrylate monomers (MTAs) and their corresponding polymers (PMTAs) bearing 1,3-thiazole and 1,2,3-triazole groups with different spacer groups were designed, inspired by azole heterocycles found in nature. PMTAs were obtained by a simple synthetic approach from alkynyl alcohols and a thiazole azide derivative, followed by conventional radical polymerization. The N-alkylation of azole rings allowed the preparation of mono and dicationic polyelectrolytes (PMTAs-RI) with different amphiphilic natures. The resulting antimicrobial properties towards different microorganisms, bacteria and fungi, which are the cause of healthcare-associated infections, as well as their hemotoxic action using human blood red cells (RBCs) are reported. The results demonstrate that methyl and butyl quaternized PMTAs present a highly selective toxicity against microorganisms because they are non-hemolytic (some of them with selectivity values higher than 1000). In contrast, longer N-alkyl analogs lead to a reduction in antimicrobial activity, showing a general structure–activity relationship that depends on the amphiphilic balance of the polycation. These polymeric families may provide a scope for developing a new class of versatile antimicrobial QUATS with promising biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...