On the structure–control relationship of amide-functionalized SG1-based alkoxyamines for nitroxide-mediated polymerization and conjugation†
Abstract
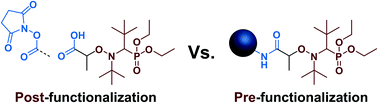
The functionalization of alkoxyamines prior to nitroxide-mediated polymerization (NMP) induces important structural variations when compared to the parent molecules. This may have important consequences on the design of functionalized materials by pre-functionalization. In this context, a wide range of amide-functionalized alkoxyamines (a functionality often obtained after conjugation from COOH– and N-succinimidyl-containing alkoxyamines) based on the nitroxide SG1 (N-tert-butyl-N-(1-diethyl phosphono-2,2-dimethylpropyl) nitroxide) have been synthesized and their dissociation rate constants (kd) have been determined. To rationalize their reactivity, a multi-parameter procedure was applied and enabled to discriminate disubstituted amide-functionalized alkoxyamines from monosubstituted ones. Monosubstituted alkoxyamines exhibited lower kd than their disubstituted counterparts (Ea increase of ∼7–10 kJ mol−1) because of the occurrence of intramolecular hydrogen bonding (IHB) between the alkyl and the nitroxide fragments. NMP of styrene, n-butyl acrylate and methyl methacrylate with a small amount of acrylonitrile was then successfully performed from two representative secondary SG1-based alkoxyamines employed for conjugation: namely AMA (COOH-containing) and AMA-NHS (N-succinimidyl derivative), and compared to polymerizations initiated with AMA-Gem, an AMA-based alkoxyamine pre-functionalized with the anticancer drug Gemcitabine (Gem) and subjected to IHB. Although AMA-NHS showed the best results due to its lower Ea, the strong polarity of the Gem moiety that counter-balanced the detrimental effect of IHB over its kd still allowed for a reasonable control.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...