Polypeptoids with tunable cloud point temperatures synthesized from N-substituted glycine N-thiocarboxyanhydrides†
Abstract
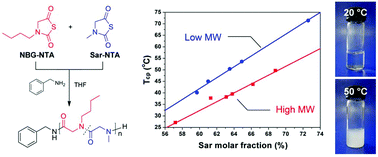
N-substituted glycine N-thiocarboxyanhydrides (NTAs) are alternative monomers for preparing polypeptoids. With an easy synthetic approach and stability during purification and storage, they have much more potential for mass production than the corresponding N-carboxyanhydrides (NCAs). Thermoresponsive copolypeptoids are synthesized by copolymerization of sarcosine NTA (Sar-NTA) with N-butylglycine NTA (NBG-NTA) initiated by benzylamine in THF at 60 °C. Polypeptoids with a degree of polymerization over 150 are obtained for the first time through primary amine-initiated NTA polymerizations. The molecular weights (MWs) and compositions of poly(sarcosine-r-N-butylglycine)s [P(Sar-r-NBG)s] are controlled by the feed molar ratios of [Sar]/[NBG]/[benzylamine]. The thermal behaviors of the copolypeptoids are investigated. Reactivity ratios of Sar-NTA and NBG-NTA are determined as 1.70(7) and 0.63(7), indicating a random distribution of the two monomers in the polypeptoid products. The structure and precise amino chain end of P(Sar-r-NBG) are confirmed by MALDI-ToF mass analysis. P(Sar-r-NBG)s have lower critical solution temperatures (LCST) and exhibit reversible phase transitions in aqueous solution. Their cloud point temperatures (Tcps) are tunable between 27 and 71 °C by adjusting the sarcosine molar fraction in copolymers. In addition, Tcp transitions depend on the MWs and the concentrations of the polypeptoids, as well as salt additives, to a certain degree. A biocompatibility study on P(Sar-r-NBG) reveals a controlled cytotoxicity related to the composition of polypeptoids. Easily accessible from NTA polymerizations, polypeptoids are therefore novel degradable materials with LCST for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...