Facile synthesis of photolabile dendritic-unit-bridged hyperbranched graft copolymers for stimuli-triggered topological transition and controlled release of Nile red†
Abstract
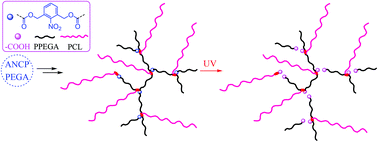
Different from hyperbranched star-like polymers and dendritic brushes, dendritic-unit-bridged hyperbranched graft copolymers (DHGCs) with branching point linked branches and linear grafts can be regarded as a new subclass of hyperbranched-graft-linear copolymers. This study aims at the synthesis and properties of photocleavable DHGCs comprising oligomeric branches composed of poly(ethylene glycol) methyl ether acrylate (PEGA) units, linear poly(ε-caprolactone) (PCL) grafts and o-nitrobenzyl ester (ONBE) moieties in the dendritic unit. Based on a multifunctional inimer 3-((2-acryloyloxymethyl-2-hydroxymethyl)propionyloxy)methyl-2-nitrobenzyl 4-cyano-4-(phenylcarbonothioylthio)pentanoate (ANCP), functional DHGCs were controllably synthesized via two step reactions. RAFT copolymerization afforded hyperbranched poly(ANCP-co-PEGA) (PAP), followed by CL polymerization to achieve PAP-g-PCL. Upon photo-cleavage, hyperbranched PAP was converted into linear polymers, and PAP-g-PCL was readily degraded into mixtures of linear, star and graft polymers. With increasing UV irradiation time, the PAP-g-PCL micelles were gradually evolved into vesicles and multicompartment vesicles due to photo-triggered cleavage and reaggregation. Upon normal and on-demand UV irradiation, the release kinetics for controlled release of Nile red from copolymer aggregates could be tuned in a wide range, revealing the great potential in smart drug delivery systems. This study affords a versatile method to construct photolabile DHGCs, which opens up a new route to explore unique properties of novel topological copolymers.

 Please wait while we load your content...
Please wait while we load your content...