Mussel-inspired protein-repelling ambivalent block copolymers: controlled synthesis and characterization†
Abstract
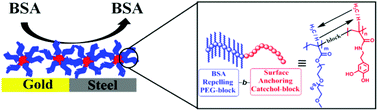
This paper describes the reversible addition–fragmentation chain transfer (RAFT) polymerization of mussel-inspired acetonide-protected dopamine (meth)acrylamide monomers (ADA and ADMA) and its implementation to the synthesis of innovative ambivalent block copolymers. They consist of a hydrophobic poly((meth)acrylamide) block functionalized by catechols and a hydrophilic segment of a poly((meth)acrylate) bearing pendent PEG chains. For the first time, a series of well-defined P(PEGAm-b-ADAn) and P(ADMAn-b-PEGMAm) diblock copolymers across a range of molar masses (13–42 kg mol−1) with low molar mass dispersities (Đ = 1.12 − 1.25) were reported. Post polymerization, trifluoroacetic acid (TFA) treatment yields block copolymers bearing free-catechol units in quantitative yields (>95%) with a slight noticeable hydrolysis of pendent-PEG units (2%–4%). The self-assembly of the amphiphilic block copolymers into spherical micelles was demonstrated by 1H NMR, DLS and TEM imaging techniques. Real-time quartz crystal microbalance with dissipation monitoring (QCM-D) studies revealed that free-catechol groups were necessary for a strong anchoring onto gold and stainless steel surfaces because acetonide-protected and catechol-oxidized block copolymers completely desorbed from the surface in the rinsing step. The ambivalent nature of catechol functionalized block copolymers was studied by bovine serum albumin (BSA) adsorption on polymer modified surfaces, which displayed improved resistance against BSA adsorption, when compared to an unmodified surface.

 Please wait while we load your content...
Please wait while we load your content...