Non-ionic water-soluble “clickable” α-helical polypeptides: synthesis, characterization and side chain modification†
Abstract
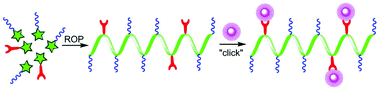
A series of water-soluble non-ionic “clickable” polypeptides has been synthesized by organo-initiated ring-opening co-polymerization (ROP) of γ-propargyl-L-glutamic acid N-carboxyanhydride (PLG NCA) and N-ε-2-[2-(2-methoxyethoxy)ethoxy]acetyl-L-lysine N-carboxyanhydride (EG2-LYS NCA). The pendant alkyne side groups can be modified with azido-containing hydrophobic and hydrophilic bioactive moieties, producing polypeptide conjugates with good water solubility. Circular dichroism (CD) reveals that both the parent polypeptides and the modified polypeptide conjugates maintain high levels of α-helical conformations in aqueous solutions. A preliminary cell study indicated the cell binding peptide GRGDS (Gly-Arg-Gly-Asp-Ser) modified copolymers are able to induce integrin-mediated cell adhesion.

 Please wait while we load your content...
Please wait while we load your content...