Facile synthesis of well-defined redox responsive diselenide-labeled polymers via organoselenium-mediated CRP and aminolysis†
Abstract
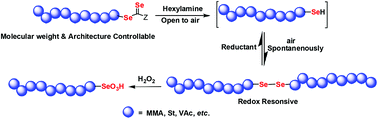
Diselenide-containing polymer is an attractive polymer for their redox sensitivity, and has potential applications in bio-related areas. In this work, the synthesis of diselenide (Se–Se)-labeled polymers based on diselenocarbonate-mediated controlled radical polymerization (CRP) was investigated. Diselenocarbonate-end capped polymers from diselenocarbonate-mediated CRP were transformed to diselenide-centered polymers through high-efficiency aminolysis and a spontaneous oxidation coupling reaction in an open system. The process of aminolysis and spontaneous oxidation of the polymer chain ends was monitored by UV-Vis, GPC and NMR characterizations. The obtained diselenide-centered polymers showed reversible redox-responsive behavior. This work provides a protocol for introducing a redox responsive Se–Se bond into the polymer backbones. Importantly, the molecular weight and architectures of the diselenide-containing polymer can be well defined, which would be potentially useful for the fabrication of bio-related polymeric materials.

 Please wait while we load your content...
Please wait while we load your content...