Accelerated living cationic ring-opening polymerization of a methyl ester functionalized 2-oxazoline monomer†
Abstract
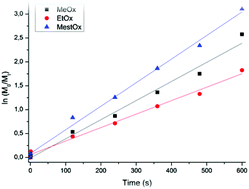
Kinetic studies on the homo- and copolymerization of 2-methoxycarboxyethyl-2-oxazoline (MestOx) with 2-methyl-2-oxazoline (MeOx) and 2-ethyl-2-oxazoline (EtOx) were performed. For the homopolymerisation of MestOx an increased propagation rate constant was observed compared to MeOx and EtOx while the copolymerization of MestOx with MeOx or EtOx unexpectedly revealed slower incorporation of MestOx. Density functional theory (DFT) calculations show that nearby MestOx residues in the living chain can activate both the oxazolinium chain end and the attacking monomer, stabilizing the propagation transition state, leading to faster homopolymerisation of MestOx. These effects also accelerate incorporation of both monomers in the copolymerisations. However, since MeOx is shown to be more nucleophilic than MestOx, the incorporation order is reversed in the copolymerisations.


 Please wait while we load your content...
Please wait while we load your content...