A facile and versatile strategy to efficiently synthesize sulfonated poly(butylene succinate), self-assembly behavior and biocompatibility†
Abstract
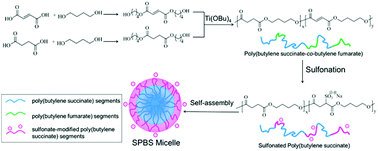
A series of amphiphilic and anionic copolyesters of sulfonated poly(butylene succinate) (SPBS) with sulfonate groups distributed randomly along the biodegradable backbone were synthesized via addition of sodium hydrogen sulphite to carbon–carbon double bonds on the backbone of poly(butylene succinate-co-butylene fumarate) (PBSF). The content of hydrophilic sulfonate groups can be facilely regulated by changing the initial feed ratio. The structures of PBSF and SPBS were systematically characterized by NMR and GPC. The negatively charged micelles self-assembled from SPBS were prepared by the dialysis method and characterized by NMR, DLS and TEM. In vitro cytotoxicity assay indicates that the SPBS micelles possess excellent biocompatibility. The biocompatibility of SPBS micelles increases with increasing content of sulfonate groups. This work provides a broad new method to facilely synthesize novel anionic copolyesters with high efficiency and controllable anion content. These copolyesters are highly promising as drug delivery carriers for cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...