A light-regulated bZIP module, photozipper, induces the binding of fused proteins to the target DNA sequence in a blue light-dependent manner†
Abstract
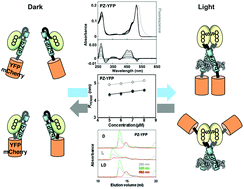
Aureochrome-1 (AUREO1) has been identified as a blue light (BL) receptor responsible for the BL-induced blanching of a stramenopile alga, Vaucheria frigida. BL induces the dimerization of monomeric AUREO1, which subsequently increases its affinity for the target sequence. We made a synthetic gene encoding N-terminally truncated monomeric AUREO1 (Photozipper protein) containing a basic region/leucine zipper (bZIP) domain and a light-oxygen-voltage-sensing domain. In the present study, yellow fluorescent protein or mCherry protein was fused with the Photozipper (PZ) protein, and their oligomeric structures and DNA-binding were compared in the dark and light states. Dynamic light scattering and size exclusion chromatography demonstrated that the hydrodynamic radii and molecular masses of the fusion proteins increased upon BL illumination, suggesting that fusion PZs underwent BL-induced dimerization. Moreover, BL-induced dimerization enhanced their affinities for the target sequence. Taken together, PZ likely functions as a BL-regulated bZIP module in fusion proteins, and can possibly provide a new approach for controlling bZIP transcription factors.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...