A method for investigating the stereochemical course of terpene cyclisations†
Abstract
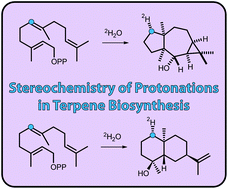
Three sesquiterpene cyclases from Streptomyces scabei 87.22, Streptomyces venezuelae ATCC 10712 and Streptomyces clavuligerus ATCC 27064 were characterised and their products were identified as (−)-neomeranol B, (+)-isodauc-8-en-11-ol and (+)-intermedeol, respectively. The stereochemical courses of the terpene cyclisations were investigated by use of various 13C-labelled FPP isotopomers. A quick and easy test was developed that allows to distinguish reprotonations of olefinic double bonds in neutral intermediates from the two stereoheterotopic faces. The method makes use of incubating 13C-FPP isotopomers labelled at the reprotonated carbon in deuterium oxide and subsequent HSQC analysis of the product. A 1,7-cyclisation towards (+)-isodauc-8-en-11-ol was followed by use of (1,7-13C2)FPP. Surprisingly, the (+)-isodauc-8-en-11-ol also accepted (2Z,6E)-FPP resulting in the same product profile as obtained from (2E,6E)-FPP.

 Please wait while we load your content...
Please wait while we load your content...