Enantioselective oxidative boron Heck reactions
Abstract
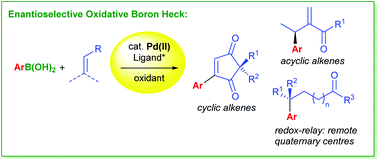
This review highlights the use of the oxidative boron Heck reaction in enantioselective Heck-type couplings. The enantioselective oxidative boron Heck reaction overcomes several limitations of the traditional Pd(0)-catalysed Heck coupling and has subsequently allowed for intermolecular couplings of challenging systems such as cyclic enones, acyclic alkenes, and even site selectively on remote alkenes.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...