Divergent and convergent synthesis of GalNAc-conjugated dendrimers using dual orthogonal ligations†
Abstract
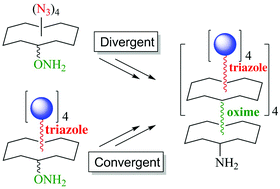
The synthesis of glycodendrimers remains a challenging task. In this paper we propose a protocol based on both oxime ligation (OL) to combine cyclopeptide repeating units as the dendritic core and the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) to conjugate peripheral α and β propargylated GalNAc. By contrast with the oxime-based iterative protocol reported in our group, our current strategy can be used in both divergent and convergent routes with similar efficiency and the resulting hexadecavalent glycodendrimers can be easily characterized compared to oxime-linked analogues. A series of glycoconjugates displaying four or sixteen copies of both α and β GalNAc have been prepared and their ability to inhibit the adhesion of the soybean agglutinin (SBA) lectin to polymeric-GalNAc immobilized on microtiter plates has been evaluated. As was anticipated, the higher inhibitory effect (IC50 = 0.46 μM) was measured with the structure displaying αGalNAc with the higher valency (compound 13), which demonstrates that the binding properties of these glycoconjugates are strongly dependent on the orientation and distribution of the GalNAc units.
- This article is part of the themed collection: Multivalent Biomolecular Recognition

 Please wait while we load your content...
Please wait while we load your content...