Novel sirtuin inhibitory warheads derived from the Nε-acetyl-lysine analog l-2-amino-7-carboxamidoheptanoic acid†
Abstract
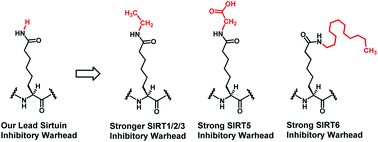
Built upon the catalytic mechanism-based pan-SIRT1/2/3 inhibitory warhead L-2-amino-7-carboxamidoheptanoic acid (L-ACAH, a close structural analog of Nε-acetyl-lysine) that our laboratory discovered recently, in the current study, its carboxamide NH2-ethylated analog was found to be a ∼2.4–6.6-fold stronger SIRT1/2/3 inhibitory warhead than L-ACAH. Carboxamide NH2-dodecylated and carboxymethylated analogs of L-ACAH were also identified as potent SIRT6 and SIRT5 inhibitory warheads, respectively.

 Please wait while we load your content...
Please wait while we load your content...