Copper-catalyzed cyanation of aryl iodides with α-cyanoacetates via C–CN bond activation†
Abstract
A Cu(I)-catalyzed cyanation reaction of aryl iodides with α-cyanoacetates is reported herein, which uses α-cyanoacetates as the nontoxic and easy-handling CN source through copper-mediated C–CN bond cleavage. This reaction enables access to aryl nitriles with an array of functional groups on the aromatic ring in good to excellent yields.
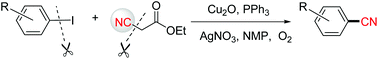

 Please wait while we load your content...
Please wait while we load your content...