3D shapes of aryl(dihydro)naphthothiophenes: a comprehensive and structural study†
Abstract
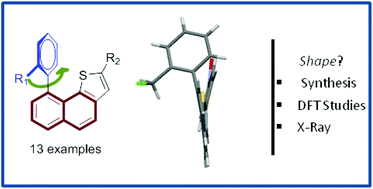
Convenient access to new aryl(dihydro)naphthothiophenes is described using a common β-chloroacrolein derivative. Our strategy is based on the construction of a condensed thiophene ring prior to a Suzuki–Miyaura coupling and allowed installing various substituents at the molecular platform. The overall shapes of these architectures were confirmed by X-ray analyses and were in good agreement with theoretical calculations. It has been established that the relative orientation between all fragments that composed molecules within this series is strongly related to both steric and electronic factors. Contribution of these key parameters revealed to be crucial to rationalize attempts to prepare fluorenone and fluorene derivatives from aryl(dihydro)naphthothiophene platforms.

 Please wait while we load your content...
Please wait while we load your content...