Recent advances in the ruthenium-catalyzed hydroarylation of alkynes with aromatics: synthesis of trisubstituted alkenes
Abstract
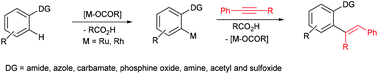
The hydroarylation of alkynes with substituted aromatics in the presence of a metal catalyst via chelation-assisted C–H bond activation is a powerful method to synthesize trisubstituted alkenes. Chelation-assisted C–H bond activation can be done by two ways: (a) an oxidative addition pathway and (b) a deprotonation pathway. Generally, a mixture of cis and trans stereoisomeric as well as regioisomeric trisubstituted alkenes was observed in an oxidative addition pathway. In the deprotonation pathway, the hydroarylation reaction can be done in a highly regio- and stereoselective manner, and enables preparation of the expected trisubstituted alkenes in a highly selective manner. Generally, ruthenium, rhodium and cobalt complexes are used as catalysts in the reaction. In this review, a ruthenium-catalyzed hydroarylation of alkynes with substituted aromatics is covered completely. The hydroarylation reaction of alkynes with amide, azole, carbamate, phosphine oxide, amine, acetyl, sulfoxide and sulphur directed aromatics is discussed.

 Please wait while we load your content...
Please wait while we load your content...