Regio- and stereoselective synthesis of 2′-β-substituted-fluoroneplanocin A analogues as potential anticancer agents†
Abstract
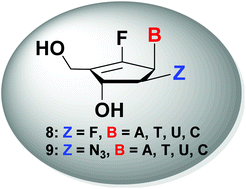
A series of 2′-β-substituted-6′-fluoro-cyclopentenyl-pyrimidines and -purines 8 and 9 were successfully synthesized from D-ribose in a regio- and stereoselective manner. The functionalization at the C2-position of 6′-fluoro-cyclopentenyl nucleosides was achieved via regioselective protection of a hydroxyl group at the C3-position and stereoselective formation of C2-triflate followed by direct SN2 reaction with a fluoro or azido nucleophile. All the synthesized compounds were evaluated for their anticancer activities in several tumor cell lines, but were found to be neither active nor toxic.

 Please wait while we load your content...
Please wait while we load your content...