Regioselective functionalisation of dibenzothiophenes through gold-catalysed intermolecular alkyne oxyarylation†
Abstract
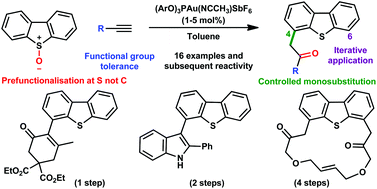
A protocol has been developed for direct Csp3–Csp2 bond formation at the 4- and 6-positions of dibenzothiophenes using a gold(I) catalyst with terminal alkynes and dibenzothiophene-S-oxides. The sulfoxide acts as a traceless directing group to avoid the need to prefunctionalise at carbon. The iterative use of this protocol is possible and has been employed in the preparation of novel macrocyclic structures. In addition, a cascade process shows how oxyarylations can be combined with other processes resulting in complex, highly efficient transformations.

 Please wait while we load your content...
Please wait while we load your content...