Unraveling the intramolecular cyclization mechanism of oxidized tryptophan in aqueous solution as a function of pH†
Abstract
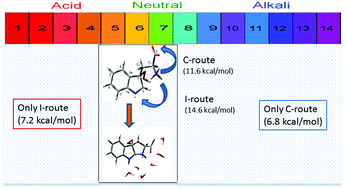
The aqueous intramolecular cyclization of 3a-hydroperoxitryptophan, Trp-OOH, (an intermediate in the photodynamic treatment of cancer) is studied at the PCM-MP2/aug-cc-pVDZ//PCM-B3LYP/aug-cc-pVDZ computational level with and without explicit water molecules. The three-cycle product may evolve to the metabolite N-formyl kynurenine in living beings or can be a building block in the formation of indole alkaloids in organic synthesis. When the pH is close to the isoelectric point of tryptophan, we have found two cyclization mechanisms, one passing along zwitterion intermediates, I-route (beginning with a proton transfer from the ammonium to the N-indole atom) and the other involving neutral isomers, C-route (starting with the attack of N-amino to C2-indole). At this pH, the discrete-continuum model with six explicit water molecules predicts a Gibbs energy barrier of 14.6 kcal mol−1 for the I-route and of 11.6 kcal mol−1 for the C-route. It is possible to experimentally tune the operating mechanism since in acidic environments only the I-route is available (Gibbs energy barrier of 8.4 kcal mol−1) whereas in basic media only the C-route can operate (Gibbs energy barrier of 6.7 kcal mol−1). These data explain the trend of Trp-OOH to easily decompose under basification.

 Please wait while we load your content...
Please wait while we load your content...