Efficient routes towards a series of 5,5′-bithiazolidinylidenes as π-electron acceptors†
Abstract
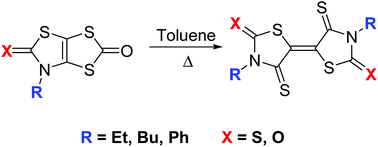
Different approaches have been studied in order to prepare efficiently the sulfur rich electron acceptor, DEBTTT. Among the various routes used, the one going through the synthesis of a bicyclic derivative, where the thiazole-2-chalcogenone is fused with a 1,3-dithiole-2-one, leads to the target molecule under milder conditions and better yield. Thus, this approach has been explored for the synthesis of a series of acceptors either by modifying the substituent on the thiazole core or by changing the exocyclic chalcogen atoms. All these sulfur rich electron acceptors exhibit short intra- and intermolecular S⋯S contacts in the solid state. Electrochemical investigations show that the nature of the exocyclic chalcogen atom of the thiazole ring has a significant influence on the accepting ability as a cathodic shift of about 220 mV is observed just by changing sulfur for oxygen. This structural modification enables the tuning of the redox properties.

 Please wait while we load your content...
Please wait while we load your content...