(E)- and (Z)-stereodefined enol phosphonates derived from β-ketoesters: stereocomplementary synthesis of fully-substituted α,β-unsaturated esters†
Abstract
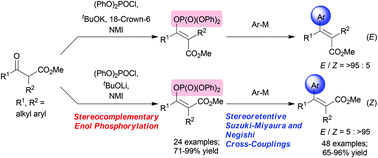
A versatile, robust, and stereocomplementary synthesis of fully-substituted (E)- and (Z)-stereodefined α,β-unsaturated esters 3 from accessible α-substituted β-ketoesters 1via (E)- and (Z)-enol phosphonates was achieved. The present method involves two accessible reaction sequences: (i) (E)- and (Z)-stereocomplementary enol phosphorylations of a wide variety of β-ketoesters 1 (24 examples; 71–99% yield, each >95 : 5 ds), and (ii) (E)- and (Z)-stereoretentive Suzuki–Miyaura cross-coupling (16 examples; 71–91% yield, >81/19 ds) and Negishi cross-coupling (32 examples; 65–96% yield, >95 : 5 ds) using (E)- and (Z)-enol phosphonates 2. 1H-NMR monitoring for a key reactive N-phosphorylammonium (imidazolium) intermediate I and an application in the synthesis of both (E)- and (Z)-tamoxifen precursors 6 are described.

 Please wait while we load your content...
Please wait while we load your content...