Nickel-catalyzed Suzuki–Miyaura type cross-coupling reactions of (2,2-difluorovinyl)benzene derivatives with arylboronic acids†
Abstract
An unprecedented highly stereoselective example of nickel-catalyzed Suzuki–Miyaura type cross-coupling reactions of (2,2-difluorovinyl)benzene derivatives with arylboronic acids was developed. The reaction proceeded efficiently in the presence of 5 mol% NiCl2(PCy3)2 and K3PO4, affording the Z-fluorostyrene derivatives in good to high yields with excellent regioselectivity.
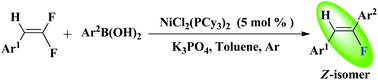

 Please wait while we load your content...
Please wait while we load your content...