Exploring carbonic anhydrase inhibition with multimeric coumarins displayed on a fullerene scaffold†
Abstract
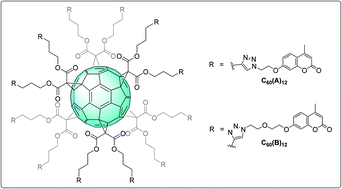
Carbonic anhydrases (CAs) are ubiquitous Zn metallo-enzymes that catalyze the reversible hydration/dehydration of CO2/HCO3−. CAs are involved in many key biological processes, therefore their inhibition has become an attractive research field. Distinct families of CA inhibitors (CAIs) have been reported, most of them interacting with the Zn(II) at the active site. Some compounds such as the coumarins are hydrolyzed before binding the entrance of the active site cavity, and thus behave as “suicide” inhibitors. This study reports the first synthesis of multimeric suicide inhibitors, designed to address the selectivity and the potency of CA multivalent inhibition. Twelve coumarin units have been grafted to a central fullerene scaffold thanks to a CuAAC reaction and the final dodecamers were assayed against 4 relevant CAs. The multimers were always stronger inhibitors than the monomeric species but no strong “multivalent effect” was found. However, our study showed that the multimeric presentation of the coumarin around the C60, indeed affected the selectivity of the relative inhibition among the 4 CAs assayed.
- This article is part of the themed collection: Multivalent Biomolecular Recognition

 Please wait while we load your content...
Please wait while we load your content...