1,8-Naphthalimide derivatives: new leads against dynamin I GTPase activity†
Abstract
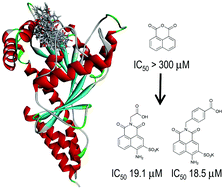
Fragment-based in silico screening against dynamin I (dynI) GTPase activity identified the 1,8-naphthalimide framework as a potential scaffold for the design of new inhibitors targeting the GTP binding pocket of dynI. Structure-based design, synthesis and subsequent optimization resulted in the development of a library of 1,8-naphthalimide derivatives, called the Naphthaladyn™ series, with compounds 23 and 29 being the most active (IC50 of 19.1 ± 0.3 and 18.5 ± 1.7 μM respectively). Compound 29 showed effective inhibition of clathrin-mediated endocytosis (IC50(CME) 66 μM). The results introduce 29 as an optimised GTP-competitive lead Naphthaladyn™ compound for the further development of naphthalimide-based dynI GTPase inhibitors.

 Please wait while we load your content...
Please wait while we load your content...