Surface-promoted aggregation of amphiphilic quadruplex ligands drives their selectivity for alternative DNA structures†
Abstract
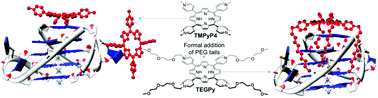
Scientists are currently truly committed to enhance the specificity of chemotherapeutics that target DNA. To this end, sequence-specific drugs have progressively given way to structure-specific therapeutics. However, while numerous strategies have been implemented to design high-affinity candidates, strategies devoted to the design of high-selectivity ligands are still rare. Here we report on such an approach via the study of an amphiphilic compound, TEGPy, that self-assembles at a liquid/solid interface to provide nanosized objects that are stable in water. The resulting aggregates, identified through atomic force microscopy measurements, were found to disassemble upon interaction with DNA in a structure-specific manner (quadruplex- versus duplex-DNA). Our results provide a fertile ground for devising new strategies aiming at concomitantly enhancing DNA structural specificity and the water-solubility of aggregation-prone ligands.

 Please wait while we load your content...
Please wait while we load your content...