Synthesis of two new enrichable and MS-cleavable cross-linkers to define protein–protein interactions by mass spectrometry†
Abstract
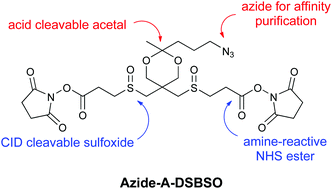
The cross-linking Mass Spectrometry (XL-MS) technique extracts structural information from protein complexes without requiring highly purified samples, crystallinity, or large amounts of material. However, there are challenges to applying the technique to protein complexes in vitro, and those challenges become more daunting with in vivo experiments. Issues include effective detection and identification of cross-linked peptides from complex mixtures. While MS-cleavable cross-linkers facilitate the sequencing and identification of cross-linked peptides, enrichable cross-linkers increase their detectability by allowing their separation from non-cross-linked peptides prior to MS analysis. Although a number of cross-linkers with single functionality have been developed in recent years, an ideal reagent would incorporate both capabilities for XL-MS studies. Therefore, two new cross-linkers have been designed and prepared that incorporate an azide (azide-A-DSBSO) or alkyne (alkyne-A-DSBSO) to enable affinity purification strategies based on click chemistry. The integration of an acid cleavage site next to the enrichment handle allows easy recovery of cross-linked products during affinity purification. In addition, these sulfoxide containing cross-linking reagents possess robust MS-cleavable bonds to facilitate fast and easy identification of cross-linked peptides using MS analysis. Optimized, gram-scale syntheses of these cross-linkers have been developed and the azide-A-DSBSO cross-linker has been evaluated with peptides and proteins to demonstrate its utility in XL-MS analysis.

 Please wait while we load your content...
Please wait while we load your content...