Cobalt(ii) catalyzed C(sp)–H bond functionalization of alkynes with phenyl hydrazines: facile access to diaryl 1,2-diketones†‡
Abstract
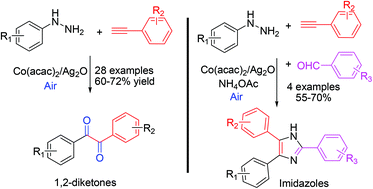
A cobalt acetylacetonate catalyzed oxidative diketonation of alkynes via C(sp)–H bond functionalization has been described. The reaction involves a free-radical mechanism, wherein the phenyl radical formed from phenyl hydrazine couples with Co(II) activated alkyne to produce 1,2-diketones. The reaction proceeds at room temperature in DMF with the use of Ag2O/air as the oxidizing system. The utility of the protocol for the synthesis of a series of imidazoles including a potent platelet aggregation inhibitor trifenagrel has been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...