Room temperature N-arylation of amino acids and peptides using copper(i) and β-diketone†
Abstract
A mild and efficient method for the N-arylation of zwitterionic amino acids, amino acid esters and peptides is described. The procedure provides the first room temperature synthesis of N-arylated amino acids and peptides using CuI as a catalyst, diketone as a ligand, and aryl iodides as coupling partners. The method is equally applicable for using relatively inexpensive aryl bromides as coupling partners at 80 °C. Using this procedure, electronically and sterically diverse aryl halides, containing reactive functional groups were efficiently coupled in good to excellent yields.
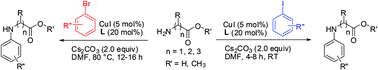

 Please wait while we load your content...
Please wait while we load your content...