Chemo-, regio-, and stereoselective Heck–Matsuda arylation of allylic alcohols under mild conditions†
Abstract
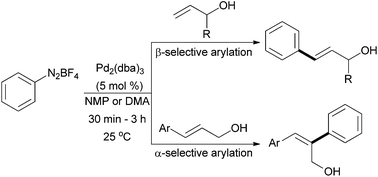
Heck arylation with allylic alcohol is extremely challenging due to chemo-, regio-, and stereoselective scrambling. Here we report a mild protocol for the alcohol selective β- and α-arylation of allylic and cinnamyl alcohols respectively with aryldiazonium salts. The steric and electronic parameters of the alkene play a prominent role in the regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...