Synthesis of 6,8,9 poly-substituted purine analogue libraries as pro-apoptotic inducers of human leukemic lymphocytes and DAPK-1 inhibitors†
Abstract
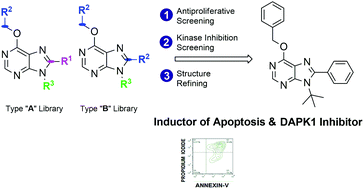
A 18-member library of 6,8,9-poly-substituted purines was prepared from pyrimidines, primary alcohols, and N,N-dimethylamides under basic conditions via a novel one-pot synthetic pathway controlled by amide sizes and the novel analogues were tested against two leukemia cell lines: Jurkat (acute T cell leukemia) and K562 (chronic erythroleukemia) cells. Compounds having a benzoxy group at C6 position of the aromatic ring exhibited antiproliferative activity in Jurkat cells whereas all compounds induced a lower effect on K562 cells. Analysis of cell cycle, Annexin-V staining, and cleavage of initiator caspases assays showed that the active purine analogues induce cell death by apoptosis. Based on these results, a new purine derivative was synthesized, 6-benzyloxy-9-tert-butyl-8-phenyl-9H-purine (6d), which displayed the highest activity of the series against Jurkat cell lines. Finally, 33P-radiolabeled kinase assays using 96 recombinant human kinases known to be involved in apoptotic events were performed. Just one of the kinases tested, DAPK-1, was inhibited 50% or more by the phenotypic hits at 10 μM, suggesting that the inhibition of this target could be responsible for the induction of cell death by apoptosis. In agreement with the phenotypic results, the most active antiproliferative agent, 6d, displayed also the lowest IC50 value against recombinant DAPK1 (2.5 μM), further supporting the potential role of this protein on the observed functional response. DAPK-1 inhibition led by 6d together with its pro-apoptotic properties against the Jurkat line makes it an interesting candidate to further investigate the role of DAPK1 kinase in triggering apoptosis in cancer cells, a role which is attracting recent interest.

 Please wait while we load your content...
Please wait while we load your content...