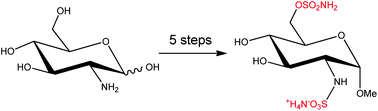
Regioselective sulfamoylation of primary hydroxyl groups enabled a 5-step synthesis (overall yield 17%) of the first reported small molecule inhibitor of sulfatase-1 and 2, ((2S,3R,4R,5S,6R)-4,5-dihydroxy-2-methoxy-6-((sulfamoyloxy)methyl)tetrahydro-2H-pyran-3-yl)sulfamic acid, which obviated the use of hydroxyl protecting groups and is a marked improvement on the reported 9-step synthesis (overall yield 9%) employing hazardous trifluoromethylsulfonyl azide. The sulfamoylation methodology was used to prepare a range of derivatives of 1, and inhibition data was generated for Sulf-2, ARSA and ARSB.